Abstract
Decision-making in animal health relies heavily on risk assessment: a transparent, systematic and science-based approach to evaluating undesirable events such as disease introduction. Both qualitative and quantitative risk assessments are commonly used to guide policies designed, for example, to prevent diseases from entering countries or regions that are free of them. Transboundary animal infections such as avian influenza, foot and mouth disease (FMD) and peste des petits ruminants (PPR) are examples where risk assessment is used for mitigating risk of introduction and guiding surveillance efforts [1-3]. In this article, we explore how artificial intelligence (AI) can complement traditional risk assessment practices in animal health, highlighting the opportunities and challenges it presents, with a particular focus on transboundary disease threats.
Risk assessment frameworks represent structured and transparent guidelines for evaluating risks. An example is the World Organisation for Animal Health (WOAH) Import Risk Analysis Framework, developed to address risks related to the importation of animals and animal products [4]. This framework is flexible enough to accommodate the complexity of real-world scenarios and the diversity of potential hazards. Its structured approach explicitly defines causal pathways supported by established disease mechanisms, and is particularly well-suited for scenario analysis and decision support. By emphasising a systematic and transparent approach, the framework ensures that risk assessments remain clear and adaptable as new information becomes available. Over the years, the WOAH Import Risk Analysis Framework has become a fundamental reference for international animal health stakeholders, guiding decisions on managing risks associated with the introduction of diseases into disease-free countries or regions [5-7].
A fundamental principle in risk assessment is transparency, which underpins the credibility and acceptance of risk management decisions. Whether assessing the risk of disease introduction, evaluating control measures or guiding surveillance strategies, transparent practices ensure that underlying assumptions, methods and data are fully documented and accessible. This transparency allows stakeholders, including policymakers, veterinarians and producers, to understand the reasoning behind decisions, thereby increasing trust in the process. Transparency is especially important in the context of international trade, where decisions concerning the importation of animals and animal products must be scientifically justified. The World Trade Organization’s Agreement on the Application of Sanitary and Phytosanitary Measures (SPS Agreement) explicitly recognises scientific risk assessment as the foundation for such decisions, stressing that measures should be transparent, science-based and impartial [8]. In the case of import risk assessments, transparency serves multiple functions: it facilitates communication, builds stakeholder trust and promotes fairness between trading partners. It also allows exporting countries to understand and, if necessary, challenge the measures imposed by importing countries. Transparency is therefore critical to risk assessment, and innovative methods driven by AI should be designed in such a way that transparency is preserved.
Integrating AI with traditional risk assessment
The rapid development and increasing accessibility of AI bring new opportunities and challenges to the field of risk assessment in animal health. On one hand, AI methods such as machine learning (ML) can improve traditional risk assessments by facilitating the integration of diverse data sources, identifying complex patterns and managing datasets with a large number of features. Such methods can also support real-time updating of risk estimates as new data become available. However, AI methods present challenges, particularly when it comes to transparency and interpretability [9]. Traditional risk assessments are typically structured around explicit disease mechanisms, making them particularly suitable for scenario analysis and evidence-based decision-making. In contrast, AI models often operate as ‘black boxes,’ as they can make accurate predictions but do not clearly show how individual factors lead to a particular outcome (Figure 1).
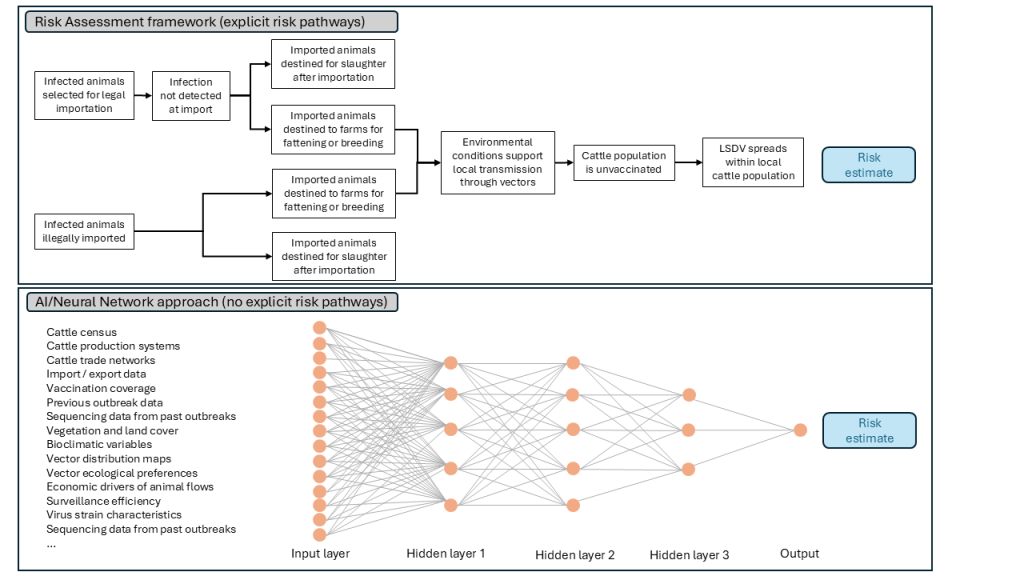
Figure 1. Outline of classical risk assessment and AI-based approaches to assessing risk of introduction and establishment of lumpy skin disease virus (LSDV) in a disease-free country, following outbreaks in a neighbouring country.
The classical approach explicitly models risk pathways, making the reasoning transparent. In contrast, the AI-based approach (a neural network) can efficiently integrate very diverse data but often acts as a ‘black box,’ making the interpretation of results less transparent. In the AI-based approach, the hidden layer processes input data to learn complex patterns linked to disease risk. While this may improve predictive performance, it limits transparency by obscuring how individual variables influence the outcome, contributing to the model’s ‘black box’ nature.

Image copyright: Igor Barilo, Getty Images
This lack of transparency raises concerns about the applicability of AI-driven models for policy-driven decisions, which require transparency and justification of outcomes. One promising approach to addressing this challenge is the integration of causal reasoning, or, more formally, causal modelling frameworks, with data-driven methods. Causal reasoning seeks to understand and make explicit the relationships between variables, such as how specific risk factors influence the probability or severity of disease outcomes.
This can ideally complement purely data-driven models, the latter of which may uncover correlations between input variables and outcomes without representing the underlying mechanisms that drive them. By combining the predictive capabilities of AI with the structured logic of causal frameworks, it becomes possible to generate models that are both powerful and interpretable [10]. In this way, we can maintain the theoretical grounding and transparency of traditional risk assessment approaches while making use of AI’s ability to process large, complex datasets and identify patterns that may not be immediately apparent.
One application is the use of ML as a preprocessing tool to identify patterns or anomalies within the data, which are then analysed through more structured, theory-driven methods. This combined approach helps preserve transparency while benefiting from computational advances. Another application where AI can make a significant contribution is dynamic risk assessment. AI models can support adaptive risk assessments that change as new data become available, combining real-time data from several sources (e.g. surveillance reports or animal movement data). In the context of this potential application, incorporating feedback into the risk assessment framework could represent a valuable advancement. Reinforcement learning algorithms, which can adapt in real time to new information, may play a role in this process, enabling more informed and timely decision-making. Such algorithms can incorporate data from various sources and adjust predictions as conditions change, making them particularly suitable for continuously evolving risk contexts [11]. This dynamic capability aligns well with risk assessments that address rapidly changing epidemiological situations, such as emerging infectious diseases. A good example is the current challenge posed by avian influenza viruses, with their constantly evolving patterns that make it especially challenging to assess and manage risk over time [12].
While these applications are promising, real-world implementations of integrated, adaptive AI approaches in animal health risk assessment are still lacking. Even in the human health domain, where data availability and digital infrastructure are often more advanced, successful examples of causal machine learning models in operational use remain scarce. As highlighted in a recent review, proof-of-concept studies are still needed before such tools can be routinely deployed [13]. However, ongoing improvements in the collection, standardisation and interoperability of animal health surveillance data are likely to make these integrated approaches increasingly feasible in the near future.
Recommendations for future risk assessment practices
The integration of AI into animal health risk assessment presents both promising opportunities and significant challenges, particularly in terms of ensuring transparency and interpreting results. To benefit from these technologies without compromising the strengths of traditional risk assessment, we need to ensure that transparency and sound theoretical foundations, grounded in real-world understanding and domain expertise, remain central. One important step is to develop tools that combine the predictive capabilities of AI with the clarity of models that are structured around causal relations. These hybrid models can enhance flexibility and performance, while maintaining the interpretability that is crucial for building trust and making evidence-based decisions.
In making AI models more transparent by incorporating elements of causal reasoning, we can ensure that their outputs are not only accurate, but also clear and meaningful for decision-makers and stakeholders. It is also important that AI tools be developed collaboratively by technical experts and those with an understanding of animal health and policy, ensuring that the tools meet real-world needs and are rooted in the realities of animal health decision-making. Furthermore, clear and consistent practices for reporting the design, assumptions and outputs of AI-driven assessments are essential for building confidence among stakeholders and allowing others to critically evaluate and build upon this work. By taking such a balanced and integrated approach, we can make risk assessment more agile and responsive to new and more complex data streams, without compromising the transparency and rigour that have long formed the foundation of animal health decision-making. In doing so, we continue to enhance a tool that has been crucial in preventing and controlling animal diseases, while also supporting safe and fair international trade.
As the global authority on animal health, WOAH is well placed to guide the responsible integration of AI into import risk assessment. This might involve revising current guidelines to incorporate new methodologies, and promoting best practices to ensure transparency and interpretability are maintained. By promoting the appropriate use of AI in risk assessment, WOAH can help ensure that innovation strengthens, rather than detracts from, the scientific foundation for safe trade.
Main image copyright: Igor Barilo, Getty Images
https://doi.org/10.20506/woah.3616
References
[1] Islam SS, Akwar H, Hossain MM, Sufian MA, Hasan MZ, Chakma S, et al. Quantitative risk assessment of transmission pathways of highly pathogenic avian influenza (HPAI) virus at live poultry markets in Dhaka city, Bangladesh. Zoonoses Public Health. 2020;67(6):658-72. https://doi.org/10.1111/zph.12746
[2] Brusa V, Durrieu M, Van Gelderen CJ, Signorini ML, Schudel A. Quantitative risk assessment of FMDV introduction in a FMD free country through bone-in beef and offal importation from a FMD free with vaccination country/zone. Prev. Vet. Med. 2023;218:105995. https://doi.org/10.1016/j.prevetmed.2023.105995
[3] Zhang S, Liang R, Yang Q, Yang Y, Qiu S, Zhang H, et al. Epidemiologic and import risk analysis of peste des petits ruminants between 2010 and 2018 in India. BMC Vet. Res. 2022;18(1):419. https://doi.org/10.1186/s12917-022-03507-x
[4] World Organisation for Animal Health (WOAH). Terrestrial animal health code. 30th ed. Paris (France): WOAH; 2022. Chapter 2.1. Import risk analysis; p. 101-5. Available at: https://doc.woah.org/dyn/portal/index.xhtml?page=alo&aloId=42814 (accessed on 4 April 2025).
[5] Coultous RM, Sutton DGM, Boden LA. A risk assessment of equine piroplasmosis entry, exposure and consequences in the UK. Equine Vet. J. 2023;55(2):282-94. https://doi.org/10.1111/evj.13579
[6] Turkson PK. Qualitative release assessment of the risk of re-introduction of HPAI H5N1 virus from neighbouring countries into Ghana via cross-border trade and movement of birds and people. Rome (Italy): Food and Agriculture Organization of the United Nations; 2009. Available at: https://fao-agris-review-search-zwcsjik2pa-uc.a.run.app/search/en/providers/122602/records/647356d208fd68d546011457 (accessed on 4 April 2025).
[7] Rozstalnyy A, Roche X, Tago Pacheco D, Kamata A, Beltran Alcrudo D, Khomenko S, et al. Qualitative risk assessment for African swine fever virus introduction: Caribbean, South, Central and North Americas. Food and Agriculture Organization of the United Nations (FAO) Animal Production and Health Papers, No. 186. Rome (Italy): FAO; 2022. 80 p. Available at: https://doi.org/10.4060/cb8748en (accessed on 4 April 2025).
[8] World Trade Organization (WTO). The WTO Agreement on the Application of Sanitary and Phytosanitary Measures (SPS Agreement). Geneva (Switzerland): WTO; 1995. Available at: https://www.wto.org/english/tratop_e/sps_e/spsagr_e.htm (accessed on 9 May 2025).
[9] Guitian J, Arnold M, Chang Y, Snary EL. Applications of machine learning in animal and veterinary public health surveillance. Rev. Sci. Tech. 2023;42:230-41. https://doi.org/10.20506/rst.42.3366
[10] Prosperi M, Guo Y, Sperrin M, Koopman JS, Min JS, He X, et al. Causal inference and counterfactual prediction in machine learning for actionable healthcare. Nat. Mach. Intell. 2020;2:369-75. https://doi.org/10.1038/s42256-020-0197-y
[11] Kraemer MUG, Tsui JL, Chang SY, Lytras S, Khurana MP, Vanderslott S, et al. Artificial intelligence for modelling infectious disease epidemics. Nature. 2025;638(8051):623-35. https://doi.org/10.1038/s41586-024-08564-w
[12] World Organisation for Animal Health (WOAH). Avian influenza. Situation reports. Paris (France): WOAH; 2025. Available at: https://www.woah.org/en/disease/avian-influenza/#ui-id-2 (accessed on 9 May 2025).
[13] Feuerriegel S, Frauen D, Melnychuk V, Schweisthal J, Hess K, Curth A, et al. Causal machine learning for predicting treatment outcomes. Nat. Med. 2024;30(4):958-68. https://doi.org/10.1038/s41591-024-02902-1







